The Cxbladder sampling system has been developed with the support of leading urologists and nurses to ensure efficiency and ease of use. The process is simple and straightforward.
Ordering: Customer support teams are on call to answer questions about the test, billing and reimbursement, while sales representatives are available for in-office support.
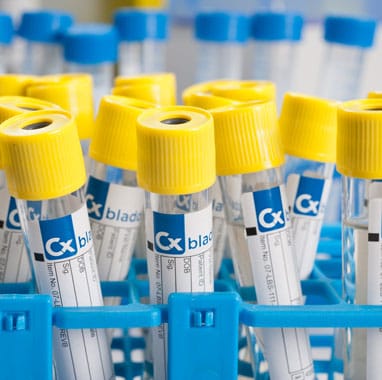
Sample Collection: A complete sampling system is provided for the collection of a patient’s urine. The sampling system contains a proprietary Cxbladder liquid (RNA preservative reagent) to stabilize the patient specimen. Only a small sample of urine is needed and no contact with urine is required.
Shipment and Analysis: The sample is sent to our CLIA-certified and CAP-accredited laboratory for analysis. Pre-paid and pre-labelled packaging is provided, and refrigeration is not required.
Analysis & Reporting: Results will be available within seven working days from sample receipt, and a detailed Cxbladder test report will be delivered securely to your practice.
In-Home Sampling is Now Available for US Patients
To simplify and streamline the bladder cancer testing process, we are now offering Cxbladder in-home sampling for surveillance patients and those presenting with hematuria across the US. Participating patients have the option of submitting their urine sample in the comfort of their own home without the need to physically visit their Urologist, though the kits must be ordered by a Physician.
Phone: 1-855-CXBLADR (1-855-292-5237)
Contact Us for More Information Download our In-Home Sampling Brochure
Support for Telemedicine & Our In-Sampling Program
1. UroToday Webinar: Alternative Strategies to Deliver Urology Care/ Bladder Cancer Investigation during COVID-19 and Beyond: Video Link
Neal Shore, MD (Medical Director, Carolina Urologic Research Center) leads discussion with Sima Porten, MD (UCSF), and Thomas Nifong, MD, (Pacific Edge Diagnostics USA).
2. Urology Times Webinar: How the COVID-19 Pandemic Impacts Practice Management & Telemedicine: Registration Link
Urology Times' Jonathan Rubenstein, MD, leads discussion with John Gore, MD (University of Washington), Aaron Spitz, MD (University of California), and Eugene Rhee, MD (Kaiser Permanente). Discussion of telemedicine and urinary biomarkers begins 50.52
3. BCAN Patient Webinar: What You Should Know About Covid-19 and Bladder Cancer
Stephanie Chisolm, Director of Education & Research at the Bladder Cancer Advocacy Network (BCAN) leads discussion with Sia Daneshmand, MD (Keck School of Medicine, USC); Professor Sandy Srinivas (Stanford Medicine); and Alec Koo, MD. Cxbladder specific discussion begins 28:12 (part 1), 46:50 (part 2)
4. Fierce Healthcare Article (23rd Apr): Half of physicians now using telehealth as COVID-19 changes practice operations. Read the article.
5. Bladder Cancer Matters Podcast: How COVID Changed How Bladder Cancer Doctors and Patients Interact: Podcast Link
Sima Porten, MD (UCSF) and Bladder Cancer Matters Host, Rick Bangs (BCAN) discuss how COVID has changed the way bladder cancer doctors and patients interact.
Last Updated: 19 Dec 2024 05:08 pm
Browse Our Latest Blog Articles
-
Cxbladder Clinical Studies
Cxbladder has been validated in multicenter clinical studies.
View our clinical publications -

Cxbladder sampling process
Cxbladder is a suite of non-invasive tests that is easy and straightforward to use.
Watch the instructional video here -

Talk to a Rep
Contact us by phone or email, or fill out an online form and a Cxbladder representative will get back to you.
Complete an information request form today






